PROTAC and Molecular Glues
Targeted protein degradation (TPD) has become a groundbreaking strategy in drug discovery since the last decade. This novel approach is emerging as a method for aiming at diseases such as cancer, inflammatory and immune diseases, and infections, driven by the abnormal expression of a pathogenic protein. Current research using TPD has focused on recruiting disease-causing proteins previously thought to be “undruggable” due to the inability of locating a strong binding domain for inhibition of activity. The ubiquitin−proteasome system (UPS) is a nature mechanism for cellular protein degradation and maintaining protein homeostasis, as part of the normal cellular housekeeping procedure. The UPS process involves an enzyme cascade that results in ubiquitination of the target protein of degradation. Ubiquitination is at the heart of both proteasomal and autophagy-mediated protein degradation, with E3 ligases as a critical component of the ubiquitination cascade.
There are more than 600 E3 ubiquitin ligases encoded by the human genome up to now, only a handful of them have been exploited for targeted protein degradation. Cereblon (CRBN), VHL, MDM2, DDB1, DCAF15, and SCF βTRCP as some examples. These subunits can be targeted by degraders that cause conformational changes that promote the formation of a ternary complex with the target protein. As a result, the formation of a ternary complex induces molecular proximity between the catalytic site of the E3 ligase and the target protein, leading to ubiquitin transfer and subsequent proteasomal degradation of the target protein.
Currently, there are 2 major pharmaceuticals interventions for TPD, namely Proteolysis targeting chimera (PROTAC) and Molecular Glue.
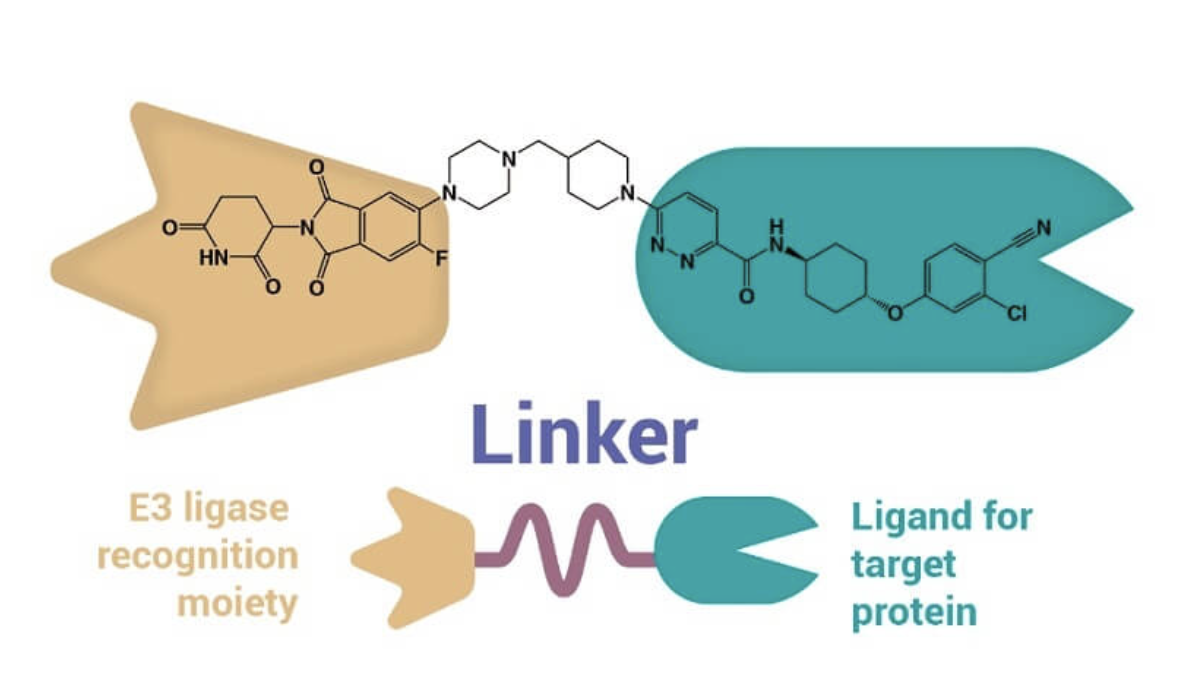
Proteolysis targeting chimera (PROTAC) is a heterobifunctional molecule composed of two active domains and a linker. PROTAC works by inducing selective intracellular proteolysis. PROTACs consist of two covalently linked protein-binding molecules: one capable of engaging an E3 ubiquitin ligase, and another that binds to a target protein meant for degradation. Recruitment of the E3 ligase to the target protein results in ubiquitination and subsequent degradation of the target protein via the proteasome. Because PROTACs need only to bind their targets with high selectivity (rather than inhibit the target protein's enzymatic activity), there are currently many efforts to retool previously ineffective inhibitor molecules as PROTACs for next-generation drugs.
Molecular glues are monovalent small molecules that reshape the surface of an E3 ligase receptor, promoting novel protein−protein interactions. Different from the PROTAC approach, in which two ligands are connected by a flexible linker that allow the two proteins to form contacts, molecular glues more directly enhance complex formation between an E3 ligase and a target protein by positioning between protein−protein interfaces and are generally defined as small molecules that interact with two protein surfaces to induce or enhance the affinity of these two proteins for each other.
Synovel Laboratory is highly experienced in TPD drug discovery projects. We offer design and synthesis of novel PROTACs including E3 ligase, linkers and ligands based on requirements such as solubility, linker distance, DMPK and toxicology.
For molecular glues, we utilized our next generation fragment-based virtual screening to design novel molecular glues candidates toward different target protein.
Because of our novel approaches, we ensure the new design will obtain Intellectual Property (IP) protection.